The last couple of decades have seen modifications and innovations being made in the production of new plastic materials. New plastic resins are being introduced, each with its unique set of properties and applicability. Materials like Polyethylene, Polypropylene, Polycarbonate, PVC, ABS, and Acetal thermoplastics are the commonly used ones.

Thermoplastics are defined as polymers that can be heated, melted, solidified over repeated cycles, leading to their recyclability. The application of heat transforms these polymers into a liquid state, thereby making them suitable for molding through modern techniques like plastic injection molding.
These thermoplastics find their applications in various industrial and commercial products and parts.
The article talks about the composition of Acetal thermoplastic, its properties, manufacturing, benefits, and applications in various sectors.
What is Acetal Thermoplastic?
Acetal thermoplastic is also known as Polyoxymethylene (POM). The resin occurs in a white semi-crystalline state. One of the reasons for its high demand in the plastic industry is its high tensile strength. Additionally, the polymer has significant creep resistance which makes it a perfect material to use as an alternative to metals for various applications.
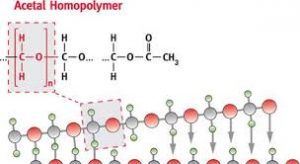
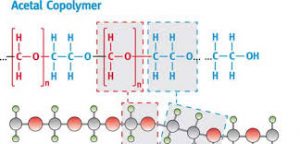
As mentioned above, Acetal or Polyoxymethylene is a thermoplastic. While thermosets have been a part of the plastic industry for a long time, the inability to reheat and reuse them has been a serious limitation. Thermoset plastics burn when heated a second time.
On the contrary, thermoplastics have a different response to heat. Thermoplastics liquify at a certain temperature and can be solidified and molded into desired shapes.
Acetal homopolymer plastic melts at 175 degree Celcius whereas Acetal copolymer melts at 162-173 degrees celsius.
Repeated heating and cooling of thermoplastics like Acetal do not result in any significant chemical degradations.
Acetal polymers exhibit repetitive and orderly structural units that give them their sharp melting points as opposed to amorphous solids.

